Introduction
Shellfish, a word colloquially used to describe edible marine animals, has a controversial and conflicting English lexicographic definition that varies in various English speaking countries such as Canada, United States, New Zealand, Australia and United Kingdom. Professor Andrew Pawley, at the Research School of Pacific and Asian Studies at the Australian National University noticed this inconsistency and conducted an informal survey based on 100 individuals from Australia, North America and New Zealand to put an end to this definition disparity (Pawley, 2004). At the end of the study, Professor Pawley redefined the word shellfish with consideration to the word variances and came up with the following definition (Pawley, 2004):
Shellfish n.
- Applied to a variable range of small edible invertebrates that live in water and that are eaten. (For most speakers not a category of fish.) The most common variant meanings are:
- Molluscs with shells, that live in water. (Usual in NZ, common in Australia and UK, rare in USA.)
- Molluscs and crustaceans. (Usual in USA, common in UK and Australia, rare in NZ, except among professionals in the food trade.)
- Edible bivalves only. (Occasional in Australia and USA.)
- Crustaceans only. (Occasional in Australia.)
- Food consisting of these animals.
- Extended by some to all members of the zoological classes listed under 1, whether eaten or not. (UK, USA, Australia, NZ.)
Contrary to the common North American description of the term shellfish, for the purpose of this investigation, we consider the order Decapoda to be strictly a non-shellfish.
Although decapods are more commonly known for their commercial purpose as seafood, there is much more to decapods than meets the stomach. This treehouse will cover a variety of information about decapods and will ultimately answer why they are one of the most unique and non-“shellfish” orders of marine metazoans out there.
Classification
The word Decapoda, created by the combination of the Greek words deka meaning ‘ten’ and pous meaning ‘foot’ is used to classify a variety of familiar marine animals such as shrimp, lobster, crayfish, hermit crabs and crabs (AskOxford.com, 2004).
The classification of the order Decapoda and overall the class Crustacea have always been a difficult task due to their morphological diversity and the lack of a fossil record amongst its species (Martin and Davis, 2001). Classification of Crustacea is based on a variety of methods including the use of cladistics, molecular systematics, developmental genetics, sperm morphology, larval morphology and fossil record (Martin and Davis, 2001).
The groups contained in the order Decapoda are presented as follows :
Eukaryotes --> Metazoa --> Bilateria --> Arthropoda --> Crustacea --> Malacostraca --> Eumalacostraca --> Caridoida --> DecapodaMajor Groups in Decapoda (Salmon, 1983)
Natantia (shrimps and prawns):
- Consists of 2000 species of crustacea.
- Found in depths that range from shallow to deep waters.
- Can be found in both salt and fresh water.
- Specialized strong and rapid swimming.
Palinura (spiny lobsters):
- Consists of 130 species of crustacea.
Astacura (lobsters and crayfishes)
Brachyura (crabs):
- Largest group of decapods containing 4500 species.
- Highly specialized morphologically with reduced antennae, small abdomen tucked under thorax, and a pair of chelae.
Anomura (mole crabs, hermit crabs, and coconut crabs):
- Consists of 1400 species.
- Abdomen is asymmetrical and well-developed.
- Last pair of walking legs are reduced in size and are oriented on the dorsal side or covered by the carapace.
- Group known for mimicking other decapod species in their form, ecology and behaviour.
Anatomy
Decapoda have many shared traits. One trait, which is implied in their name, would be their ten legs. These consist of the last five pairs of the eight pairs of thoracic appendages. Decapods are also noted for their hard exoskeleton, segmentation, and jointed appendages, which are used for sensory reception, feeding, locomotion, and defense.
The body of a decapod is divided into several distinct morphological and functional regions called tagmata. The more basal decapods have less tagmatized body plans. As this morphology is thought to be similar to that of the last common mandibulate ancestor, understanding these underlying features and their development becomes pivotal for understanding the evolution of the Decapoda.
Gills are an interesting decapod feature with an extensive surface area that is used for gas exchange with the surrounding water. These gills are located on each appendage from the second maxilliped to the fifth pereiopod.
The auxiliary heart found in decapods is a notable feature. It is located at the anterior end of the dorsal median artery before the artery branches to supply the supraesophageal ganglion (the decapod brain), and the peripheral oculomotor and visual systems. Also referred to as the cor frontale, the auxiliary heart is controlled and integrated with several other systems.
The auxiliary heart is significant due to its adaptation. Decapods possess open venous systems, an inflexible carapace, and a highly variable volume of the stomach, which all influence the blood pressure. The auxiliary heart allows decapods to better maintain blood pressure levels.
The body of a decapod contains nineteen segments, which may contain a pair of appendages, and can be divided into two main divisions: the cephalothorax (head and thorax/pereon) and the pleon (abdomen). The following is a breakdown of these features (listed from anterior to posterior).
Cephalothorax
Head
- Antennules
- Antennae
- Mandibles
- First maxillae and second maxillae
- Compound eyes, usually stalked
Pereon / Thorax
- First, second, and third maxillipeds
- The maxilliped appendages are modified to function as mouthparts. In more basal decapods, they are very similar to the pereipods.
- First, second, third, fourth, and fifth pereiopods
- Pereiopods are the primary walking legs, they are used for gathering food, and bear the sexual organs found in the fifth pereiopod in the male and the third pereiopod in the female. The gills are found in the second maxilla to the fifth pereiopod. Chelipeds are those pereipods that are armed with a chela or a claw.
Pleon / Abdomen
- First, second, third, fourth, and fifth pleopods/swimmerets
- Uropods
- Telson
Pleopods or swimmerets are used for swimming, brooding eggs (except for Natantia), catching food, sweeping food into the mouth, and sometimes bearing gills. In the males of some taxa, the first one or two pairs of pleopods are referred to as gonopods as they are specialised for fertilisation.
The tail fan is responsible for steering while swimming. Located at the posterior end of the pleon, the tail fan is composed of a pair of biramous uropods and the telson. The anus of the Decapod is located within the telson.
In crabs and other carcinised decapods, the pleon is folded under the cephalothorax

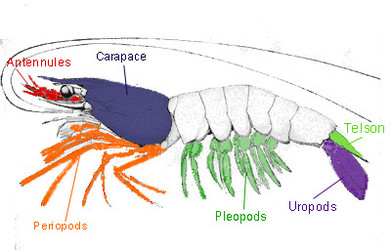
The general anatomical features of a Decapod, using Palinura as a model.
Reproduction
Decapods are egg-laying invertebrates, mainly with seperate sexes. A change of sex during the lifetime of an individual is a regular occurence in some Dendrobranchiates. In Pandalus montagui, some individuals begin life as males but then transform into functional females after 13 months.
Males of crab and lobster species are usually larger than the females and possess much larger pincers. The males also possess clasping organs used to hold the female during mating. Appendages can also be modified to assist in the sperm transfer process. The sperm is often encased in a spermatophore, and the first and second abdominal appendages are modified to transfer these spermatophores.
Methods for brooding eggs vary among the decapods as follows:
Suborder: Dendrobranchiata
- Consists of shrimp and prawns.
- Reproduce by releasing eggs into the water. Eggs then hatch as a nauplius, a larva consisting of a head and a telson.
Suborder: Pleocyemata
- Includes crabs, lobsters, and crayfish.
- Eggs are attached to abdominal appendages where special setae secrete a cement that binds the eggs to the setae. Larvae hatch in a more developed stage than a nauplius.
The Larvae
The larva of decapods and of many other crustacea is the nauplius. It consists of an unsegmented body with only a head, telson, three pairs of appendages (antennules, antennae, and mandibles), and a simple nauplius eye. Once the body is more elongated and segmented, the nauplius is called a metanauplius. The nauplius larva makes an important evolutionary statement for the crustacea as they start to diverge from other arthropods (Encyclopaedia Britannica, 2005).
Behaviour and Mechanisms
Grooming
Grooming is a common behaviour found in many crustaceans as a form of maintenance to rid their bodies of epizoic growth and particulate buildup (Bauer, 1981). In the Decapoda, this behaviour is extremely crucial and helps prevent the growth of foreign organisms on their body surfaces, loss of olfaction hairs, clogging of gills and embryo mortality. Grooming within the decapod crustaceans involves the use of chelipeds and the setal brushes at the tips of their walking legs. The most frequently groomed areas of the body include the sensory and respiratory structures that consist of the antennules, antennae, and gills (Bauer, 1981).
Mechanisms of Grooming
- Antennular Grooming:
- Preening involves the use of the third maxilipeds.
- It is the most common cleaning behaviour found amongst the order of Decapods.
- Antennal Flagellum Grooming:
- Preening method most commonly found in cardean shrimp.
- It involves the cleaning of the long chemotactile antennal flagella with setal brushes located on the first pair of chelipeds.
- Gill Cleaning:
- The enclosure of gills within the branchial chamber is a unique morphological feature found in the Decapod order of crustaceans.
- The gill chamber is flushed by reversing the respiratory current or is cleaned by the use of setae.
- General Body Grooming:
- Involves the brushing of the exoskeleton (comprised of carapace, thoracic sternites, abdominal pleurites and sternites, and body appendages) and picking at areas in between appendages and body segments.
Alternatives to Grooming
Some decapod species use other mechanisms other than grooming to maintain their body surface cleanliness. Some alternative methods include:
- Burrowing into deep sediment.
- Wedging between narrow surfaces.
- Changing temporal lifestyle from diurnal to nocturnal.
- Molting.
Reproductive Behaviour
Attraction Cues
In most decapod species, the males and females live separately from each other and pair-up as adults only to mate (Salmon, 1982). Since males and females are separated by vast spatial distances they rely on a combination of chemical, visual and acoustic signals to attract a mate (Salmon, 1982). Some of these signals are further discussed below.
Chemical Signals. This type of attraction involves sex pheromones which are the most commonly used signal in aquatic decapods (Salmon, 1982). The use of pheromones is most evident in decapod species where female molting and mating are closely linked temporally (Salmon, 1982).
Visual Signals. This type of attraction occurs most commonly in close-range courtship amongst aquatic species or long-range courtship amongst land species of decapods (Salmon, 1982). Visual signals may involve a "dance" or repetitive movement of a body part, such as "waving" (Salmon and Hyatt, 1983).
Acoustic Signals. This type of attraction occurs predominatly in ghost and fiddler crabs (Salmon and Hyatt, 1983). These acoustic "calls", that only occur during the mating season, are strictly produced by males to indirectly attract females (Salmon and Hyatt, 1983).
- Natantia (shrimps and prawns)
- Chemical attraction signals released when the female molts to signify that she is sexually receptive and ready to mate.
- Palinura (spiny lobsters)
- Chemical attractin signals released during female molting to attract male mate.
- Fertilization occurs when a male first places a spermatophore on the female's sternum, her unfertilized eggs will pass over the area and become fertilized.
- Astacura (lobsters and crayfishes)
- Courting pattern varies greatly between species.
- Courtship behaviours involve a combination of pheromones and molting depending on the species.
- Brachyura (crabs)
- Marine Species
- Species attract mates using chemical signals (e.g. sex pheromones).
- Courtship behaviour lasts longer compared to other decapod species.
- Once paired together, the male will service the female and assist in her molting. During this process, the male will protect the female from predators and other males that may try to copulate with her.
- Terrestrial Species
- Species attract mates through visual and sound courtship cues.
- Well-known example of visual courtship is that of the male fiddler crabs (genus Uca) where they "wave" their enlarged chelae to attract females to their burrow. This repetitive action has also been shown to repel other males.
- Anomura (mole crabs, hermit crabs and coconut crabs)
- Mole crab males experience neoteny and are physically smaller than females. This sexual size dimorphism allows the males and females to share the same niche therefore allowing easier access to mates during the mating season.
- Hermit crabs exhibit courtship behaviours that involve a combination of actions such as stroking, jerking, and shell-rocking.
- Marine Species
Sexual Selection
Decapods are polygynous and sexually dimorphic (Salmon and Hyatt, 1983). Males tend to be physically bigger and this allows competitive behaviour between males for access to females (Salmon and Hyatt, 1983). Females on the otherhand are selective in choosing their mates and base their decisions on the male's ability to acquire resources and the male's ability to dominate other males (Salmon and Hyatt, 1983).
Feeding
Most decapods are scavengers, feeding mostly on detritus, but it is also known that if small live fish come too close, decapods with chelae may thrust their claws after them.
Tools
It is well known that some animals such as the Monkeys use tools, e.g., sticks to get something out of reach. However, it is only well known in the scientific community that some decapods will use tools as well! There are at least two crabs that carry living sea anemones to be used as weapons. Lybia tessellata and Lybia leptochelis are two species that seem to be most unhappy without at least one sea anemone. The sea anemone Triactis producta seems to always be the anemone of choice as it is bestowed with nettle-cells. Any small animal that comes into contact with these cells would likely soon perish. The claws of Lybia are especially adapted for grasping the sea anemone's fleshy body. If live fish, amphipods, or any other of the like come too close, the crab thrusts out the sea anemone. The victim, if struck, will fall to the sea floor where it will be picked up by the crab. Hermit crabs have also been seen keeping a sea anemone on their shell for protection.
Locomotion
Locomotion of crustaceans is very important in a variety of activities including for feeding, courtship displays, grooming and walking (Evoy and Ayers, 1982). The movement of crustacean limbs involves a network of muscles, neurons and appendages used to coordinate the movement of appendages that make locomotion possible (Evoy and Ayers, 1982).
Muscles within crustaceans are involved in different types of movements where the muscles used can be classified as being synergistic or antagonistic (Evoy and Ayers, 1982). Synergistic muscles involve a single type of movement, such as walking forward (Evoy and Ayers, 1982). While anatagonistic muscles involve a variety of movements such as sideways walking, swimming, or courtship displays (Evoy and Ayers, 1982). The jointed appendages supported by muscles are highly specialized to various types of movements (Lochhead, 1961). Also, Decapod appendages are arranged in a fashion where their legs hang to allow maximum stability for their unique morphology (Lochhead, 1961). This appendage arrangement allows legs located on the posterior region to be highly specialized in pushing, while legs in the anterior region are specialized in pulling (Lochhead, 1961). Legs that are intermediate can be used for either pushing or pulling (Lochhead, 1961).
Types of Locomotion
Swimming. There are two forms of swimming associated with decapod crustaceans (Evoy and Ayers, 1982). One that involves rhythmic extension and flexing of muscles contained in the abdomen to allow jet propulsion through the water (Evoy and Ayers, 1982). The other form of swimming involves rhythmic sculling movements of the appendages (Evoy and Ayers, 1982). The mechanisms involved in swimming are expanded further below (Lochhead, 1961):
- Using appendages to act as oars
- Using appendages to create vortexes by rotational beating of the water
- Using appendages to act like propellers which move at right angles to the path of motion
- Beating the abdomen therefore driving the animal forward
- Flexing the abdomen and fanning the tail
Walking Forward. The forward walking motion for decapods involves the coordinated pushing and pulling of paired legs moving at a rhythmic out-of-phase pattern (Lochhead, 1961).
Walking Sideways. This type of locomotion is commonly found among crustaceans that have fewer than six pairs of legs (Lochhead, 1962). This special physiological adaptation allows individuals to exhibit less instances of appendages overlapping during walking (Lochhead, 1961). Sideways walking also involves the coordinated flexing and extending of legs at either sides of the body (Lochhead, 1962).
Cell Division and Cleavage Patterns
Cell cleavaging is an important and crucial step in the development of any animal especially during embryogenesis. This ubiquitous process is different in many metazoan groups and ultimately can be used to classify various groups of metazoans.
During embryo development, the decapod crustaceans undergo protostomic development where the mouth is the first developmental opening in the blastopore. The second opening then develops into the anus of the embryo. This protostomic development is also commonly found in other arthropods and in other groups such as annelids and mollusks.
Most malacostracan crustaceans undergo superficial cleavage, however the Decapoda undergo complete holoblastic cleavage. Due to the spiral holoblastic cleaving of decapod cells, they characteristically have larger eggs than other crustacea, alongside with a greater yolk mass, intralecithal cleavage, blastoderm formation and epimorphic later development (Anderson, 1973). This characteristic spiral cleaving is different from the spiral cleaving noticed in annelids (Anderson, 1973).
Biogeography
Dispersion and vicariance events are known to be responsible for the creation of faunal provinces or regions with similar groups of organisms. Physical barriers, such as ocean currents and land masses can seperate organisms allowing them to evolve away from each other. Fauna found in close proximities have been noted as being more similar (Feldman and McLay, 1993)
Different vicariance effects influencing the Decapoda
- Decapod faunas of eastern and western South America are noticeably different from each other due to barriers such as ocean current patterns.
- In the early Eocene, a strong similarity was demonstrated in decapod faunas between New Zealand and Antarctica. This similarity disappeared over time as New Zealand diverged from Antarctica due to tectonic movement.
- A noticeable similarity between the decapoda of the North American east coast and those of southern Europe suggests ocean currents as a dispersal mechanism from east to west across the Atlantic
- A Tethyan distribution pattern is evident in decapods due to similarities between Eocene faunas of Europe and Japan. The tethyan trench formed 200 million years ago in the early Jurassic and is likely responsible for moving Africa and India towards Eurasia.
Decapoda of the North Pacific region can be assigned to five major origins. These are:
- a North Pacific origin
- a Tethyan origin
- a North Polar origin
- an orgin from the high southern latitudes
- an amphitropical origin
Domestication of Decapoda
Decapods are becoming more and more popular as pets in aquaria. More stores are now carrying them as popularity increases.
Natantia
- Shrimp are kept as pets in aquaria so they will feed on detritus or algae, or they may simply be kept as ornamental pets.
- The shrimp that are kept as pets include: Yamato Numa Ebi/Caridina spp., Ghost/Grass (Paleomonetes sp.), Wood/Singapore (Atyopsis sp.) , Rock/Mountain, Bumble Bee (Atydea sp.), Macrobrachium, and Neocaridina sp.
Brachyura
- True crabs are becoming more and more popular acquarium pets, feeding on dried blood worms, brine shrimp, or dried flake food.
- Crabs that are kept as pets include: Freshwater Gold Fiddler Crabs, Freshwater Red Claw Crabs.
Anomura
- Includes hermit crabs, which have been kept as pets for a relatively long time.
- Omnivorous or herbivorous and are useful in aquaria as scavengers, eating debris and algae
- Will switch shells when provided with a larger one
- Popular hermit crab species ket as pets include the Caribbean hermit crab (Coenobita clypeatus) and the Pacific hermit crab (Coenobita compressus)
- Less common species kept as pets include Coenobita brevamanus, Coenobita rugosus, Coenobita perlatus and Coenobita cavipes.
Pleocyemata
- Will eat shrimp pellets, vegetables, tropical fish food, algae wafers, and eve small fish. Crayfish kept in aquaria include red crayfish (Cherax quadricarinatus) and blue crayfish (Procambarus alleni).
Decapod Records
Largest Decapod: : Macrocheira kaempferi, the giant spider crab. Individuals can reach a diameter of 12-14 inches (30.5-35.6cm), with a claw span of 8-9 feet (2.4-2.7m). There is a report of a crab weighing 14 pounds and a claw span of 12 feet (3.65m).
Smallest Crab: The pea crabs of the Pinnotheridae are approximately 0.25 inches (0.635cm) across the shell.
Heaviest Decapod: Homarus americanus, Atlantic Lobster. Records exist of individuals weighing over twenty pounds. The largest record, however, belongs to a lobster weighing 42 pounds and 7 ounces. This lobster was caught in 1934 and bestowed with the nickname "Mike."



 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site