Aulacidae
John T. Jennings and Andrew R. Deans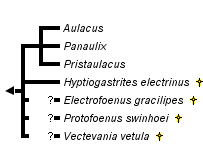

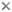
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Insects in the family Aulacidae are endoparasitoids of wood-boring wasps (Xiphydriidae) and beetles (Cerambycidae and Buprestidae) (Carlson 1979; Gauld & Bolton 1996; Smith 2001, Jennings & Austin 2004). Although at various times Aulacidae has been treated as a subfamily or other group within Evaniidae, recent literature regards them as a distinct family (e.g., Mason 1990; Naumann 1991; Gauld 1995; Jennings & Austin 2000; Smith 2001; Jennings et al. 2004a,b,c). There are three described extant genera: Aulacus and Pristaulacus, which are worldwide (except New Zealand and Madagascar) in distribution, and Panaulix, which is confined to sub-Saharan Africa. As well, the extinct Hyptiogastrites electrinus Cockerell, 1917, from Lower Cretaceous (Upper Albian) Burmese amber is included within the Aulacidae.
Smith (2001) provided a catalogue of the world aulacid fauna and included 48 species of Aulacus Jurine, 106 species of Pristaulacus Kieffer, and two species of Panaulix Benoit. Since then several new species from Australia and New Caledonia have been described, including 9 Aulacus spp. and 3 Pristaulacus spp. (Jennings et al. 2004a,b,c). About 40 more undescribed species from Australia, seven from New Guinea (Jennings, unpublished) and many more undescribed species from South America (Smith, unpublished) have been identified.
Characteristics
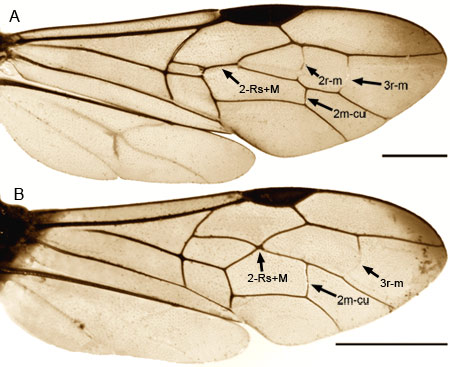
As with all Evanioidea, Aulacidae are characterised by the dorsal articulation of the metasoma on the propodeum, and are readily distinguished from evaniids and gasteruptiids by the presence of fore wing cross-vein 2m-cu (Gauld & Bolton 1996) (Figs. 1A-B), cross-vein 3r-m (Konishi 1990) (Figs. 1A-B), and a metapostnotum (Fig. 2).

Figures 1A-B. Wings of females of (1) Aulacus houstoni Jennings et al. (2) Pristaulacus davisi Jennings et al., from Western Australia. Scale bars = 1 mm. © John T. Jennings.
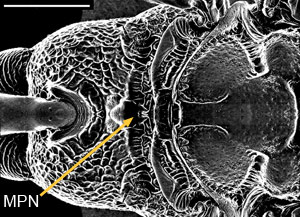
Figure 2. Dorsal view of scutellum of Aulacus douglasi Jennings et al. female from Western Australia; MPN = metapostnotum. Scale bar = 1 mm. © John T. Jennings.
Eyes small, circular or subcircular, remote from the mandibles (Fig. 3); antenna 14-segmented in female, 13-segmented in male; antennal insertions low on face, near lower margin of eyes (Fig. 3); scape usually deeply convex ventrally in lateral view, much thicker than pedicel and flagellomeres (Fig. 3); sub-antennal groove or depression to accommodate scape (Fig. 3); metapostnotum between propodeum and metanotum as a distinct sclerotisation (Fig. 2); propodeum pyramidal, metasoma inserted high on the apex; metasomal T1 and T2 fused dorsally; hind coxa usually with groove or notch on inner lateral surface, the apposed grooves or notches forming an ovipositor guide (Figs. 4A-B); hind trochanter with a transverse trochanteral groove (Fig. 5), prefemur (trochantellus) present (Fig. 5); each tarsal claw with one basal tooth (sometimes difficult to see); fore wings not plicate at rest; fore wing vein 2m-cu present (Figs. 1A-B), vein 2r-m usually present, largely spectral, vein 3r-m present, often largely spectral (Figs. 1A-B); fore wing vein 2-Rs+M either long (Fig 1A) or very short (Fig. 1B); ovipositor exserted, protruding well beyond apex of metasoma (see title image).

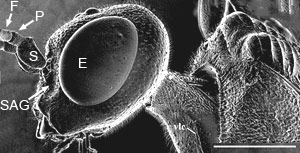
Figure 3. Lateral head and scutum of Pristaulacus mouldsi Jennings et al. female from Western Australia. F = flagellomere, P = pedicel, S = scape, E = eye, SAG = sub-antennal groove. Scale bar = 1 mm. © John T. Jennings.
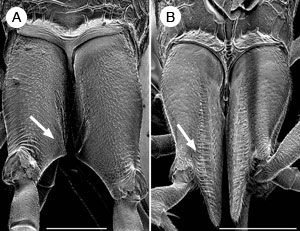
Figures 4A-B. Dorsal view of hind coxae with ovipositor guide (arrowed) of (A) Aulacus coracinus Jennings et al. and (B) A. emineo Jennings et al., both female and both species from New Caledonia. Scale bar = 500 µm for A; 200 µm for B. © John T. Jennings.
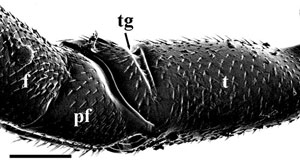
Figure 5. Lateral view of hind trochanter, prefemur, and femur of A. douglasi Jennings et al. female from Western Australia. tg= trochanteral groove, pf = prefemur (trochantellus), f = femur. Scale bar = 200 µm. © John T. Jennings.
Discussion of Phylogenetic Relationships
There are no extensive published phylogenies of Aulacidae, but there seems to be little morphological evidence that the two main aulacid genera, Aulacus and Pristaulacus, are monophyletic.
Although the wing venation of the extinct H. electrinus is identical to the majority of extant Hyptiogastrinae (Gasteruptiidae), phylogenetic analysis by Jennings et al. (2004d) placed H. electrinus as the sister taxon to the Aulacidae s.str., i.e. Aulacus , Pristaulacus. Rather than erecting a new monotypic family, Jennings et al. (2004d) included this species within a slightly more broadly defined Aulacidae. Characters that align this species with the Aulacidae include having small circular eyes, percurrent Y-shaped notauli, pyramidal shape of the propodeum, and the presence of a groove or ovipositor guide on the hind coxae.
The three other fossil taxa listed in the tree above, Protofoenus swinhoei, Electrofoenius gracilipes, and Vectevania vetula, have not been analysed phylogenetically, and their placement within Aulacidae remains uncertain (see Nel et al. 2004).
Host Relationships and Larval Biology
Aulacidae are thought to be endoparasitoids of wood boring Hymenoptera or Coleoptera (Carlson 1979; Gauld and Bolton 1996). For Aulacus, host data indicate that they mostly parasitise xiphydriid wasps in the northern hemisphere and cerambycids in the southern hemisphere. There are three documented exceptions to this; A. striatus Jurine has been bred from Xylotrechus capricornis (Gebler) (Coleoptera: Cerambycidae) in Europe (Sedivy and Capek 1988), while there are doubtful records of A. striatus from Purpuricenus koehleri L. (Cerambycidae) (Giraud 1877; Gaulle 1908) and A. aneurus Walkley from Dendroctonus (Coleoptera: Scolytidae) in New Mexico (Walkley 1952; Muesebeck 1958; Carlson 1979). Since there are no museum specimens of the latter species reared from Dendroctonus, Carlson (1979) considered the record to be doubtful. Smith (1996) suggests that both A. schiffi Smith and A. impolitus Smith probably parasitise wood boring Coleoptera, but no host records are available.
When parasitising Xiphydriidae, aulacids are recorded only from the genus Xiphydria. This host family is a small group of wood boring sawflies that are found in most temperate and tropical forest regions, except in Africa (Benson 1954; Riek 1955; Smith 1976). The larvae bore into dead or dying branches and small limbs of deciduous broad-leaved trees. Many tropical species are rarely encountered (Smith 1976), as is the case for Australian species (Riek 1955). Because of the scarcity of xiphydriid hosts, preliminary data for Australia suggests that Aulacus parasitise alternative hosts (e.g. Cerambycidae) that have a similar biology to the xiphydriids, i.e. boring into wood.
Pristaulacus, with the exception of one species, are recorded as parasitoids of various wood-boring beetles. Both Oriental and Nearctic species parasitise jewel beetle (Buprestidae) and/or longhorn beetle (Cerambycidae) larvae. For example, P. editus (Cresson) and P. californicus (Townes) parasitise buprestid and cerambycid larvae that are often found in pine cones (Townes 1950). Cerambycids are the only wood boring beetles recorded as hosts of Pristaulacus in the Australian region. For the Palaearctic, apart from cerambycids and buprestids, Pristaulacus has been recorded as parasitic on false powderpost beetles (Bostrichidae) and checkered beetles (Cleridae). Pristaulacus bimaculatus Kieffer has been reared from the timber of Ceratonia siliqua (carob) infested with Scobicia pustulata F. (Bostrichidae) and Denops albofasciata Charpentier (Cleridae) (Oehlke, 1983). Whether they are the real hosts has yet to be demonstrated. Pristaulacus patrati Serville is recorded as a parasitoid of Xiphydria annulata Jurine (Lichtenstein and Picard 1918; Györfi 1964; Oehlke 1983) and X. longicollis Geoffroy (Schimitschek 1974). Nothing is known about the host biology of Panaulix.
During oviposition, aulacids raise the metasoma, and the ovipositor is more or less directed antero-ventrally so that the valves pass between the hind coxae and through a guide formed by apposed grooves or notches on the inner surface of the hind coxae (Townes 1938, 1951; Skinner and Thompson 1960; Naumann 1991). Sometimes the guide is longitudinal as in A. emineo Jennings et al. from New Caledonia (Fig. 4B), the Nearctic A. burquei (Provancher), A. digitalis (Townes), A. lovei (Ashmead), and A. pallipes Cresson, and the type species for the genus, A. striatus Jurine, from the Palaearctic (Smith, pers. comm., Jennings et al. 2004c. However, some Aulacus, mostly those with short ovipositors, do not have coxal grooves (Townes 1951). There is no information on oviposition in Pristaulacus, but they have oviposition guides on the hind coxae similar to Aulacus (Crosskey 1951; Naumann 1991). It is likely that they have similar oviposition behaviour since their hosts are also wood boring larvae.
Aulacus have been associated with a wide range of tree species, reflecting those trees infested by their hosts. In Europe, their xiphydriid hosts (Xiphydria spp.) lay their eggs in bark crevices of a range of deciduous angiosperms including Acer (maple) (Schimitschek 1974), Alnus (alder) (Skinner and Thompson 1960; Schimitschek 1974; Evenhuis and Vlug 1975; Carlson 1979; Oehlke 1983), Betula (birch) (Schimitschek 1974; Oehlke 1983), Ostrya (hop hornbeam) (Schimitschek 1974), Populus (poplar) (Schimitschek 1974; Oehlke 1983), Quercus (oak) (Schimitschek 1974), Salix (willow) (Schimitschek 1974; Evenhuis and Vlug 1975; Oehlke 1983) and Ulmus (elm) (Schimitschek 1974). In North America, host trees include Acer, Alnus, Betula, Celtis (hackberry), Carpinus (hornbeam), Fagus (beech), Malus (crab-apple), Populus, Quercus, Tilia (lindens and limes), and Rhus (sumac) (Townes 1951; Smith 1979, 1996, 2001).
Female Aulacus striatus locate the egg-shaft of xiphydriid wasps, insert the ovipositor and lay single eggs in as many host egg batches as can be reached (Skinner and Thompson 1960). Aulacus burquei (Provancher) also lays its eggs in a similar manner (Deyrup 1984). Aulacus striatus lacks any muscular control of the ovipositor and uses movement of the body and metasomal muscles to manoeuvre the ovipositor. The ovaries of A. striatus contain some 200 eggs which, when mature, are pedunculate and 0.6 mm in length (Bugnion and Popoff 1911; Crosskey 1951).
When the host xiphydriid larva hatches, it contains a tiny larva of A. striatus (Skinner and Thompson 1966). Aulacus striatus is a typical koinobiont, delaying its development until the host xiphydriid larva has fed for almost a year and is close to the wood surface (Skinner and Thompson 1960). The aulacid larva then proceeds to feed rapidly, kills the host by feeding on its internal organs, emerges from the host skin, and spins a cocoon in which it pupates (Skinner and Thompson 1960). Askew (1971) confirmed that aulacid larvae emerge from fully grown Xiphydria larvae. The cocoon is reddish pink (Ratzeburg 1852), is fine, translucent and testaceous, and has the remains of the host larva attached (Sedivy and Capek 1988). The adult emerges about two weeks later, escaping by gnawing a hole through the thin cap of wood left by the host. Aulacus burquei also spins a cocoon (Deyrup 1984). No information is available for any Pristaulacus spp.
The larva of only one aulacid species, A. striatus, has been described in any detail. It has an incomplete, sclerotised epistoma with two dorso-lateral projections that extend to the frontal area; large, unsclerotised tridentate mandibles with a short sclerotised blade; disc-shaped maxillary and labial palps and antennae; labral, stipital and labial sclerites are present (Short 1952, 1959; Finlayson and Hagen 1977).
Collecting data show that species of Pristaulacus have been reared from a wide range of both gymnosperm and angiosperm trees. Again, these associations reflect the plant species infested by their hosts. For example, in North America, P. bilobatus (Provancher) has the same distribution as Tsuga canadensis (L.) Carr. (eastern hemlock) and the distribution of P. pacificus (Cresson) parallels that of Pseudotsuga menziesii (Mirb.) Franco (Douglas-fir) (Townes 1950).
Adult Biology
Virtually no information is available on the mating behaviour of aulacid wasps, although Skinner and Thompson (1960) describe the courtship and mating of A. striatus. Similarly, little is known of the internal anatomy of aulacids, although the digestive system and ovaries of A. striatus have been described (Bugnion and Popoff 1911). The oesophagus is long and straight, the crop dilated and the proventriculus short, striated and without a valve, while no caecae are apparent. The ventriculus is ovoid, measures 2-3 mm in length and about 1.3 mm in diameter, and is composed of small yellowish polygonal cells. There are 16 malphigian tubules. The ileum is short and without any apparent demarcation from the rectum, and four rectal plates are arranged in pairs, one anteriorly and the other posteriorly.
Although some information is available on feeding behaviour of adult aulacids for British species (Crosskey 1951), there is a lack of information for species from most other regions. Aulacus are known to visit flowers (Crosskey 1951), but whether they are feeding on pollen and/or nectar is not clear. There is nothing published on adult feeding of Pristaulacus.
Aulacus striatus have been recorded on the flowers of Apiaceae in the Palaearctic region (Crosskey 1951; Hedqvist 1973). Apiaceous plants produce pollen and also have nectariferous disks (the stylopodium) (Jessop and Toelken 1986). Aulacid mouthparts do not appear to be adapted to nectar feeding as they have a very short glossa. The mouth parts of a number of Aulacus spp. from various museum collections have been examined and found to have no pollen in or around them (Jennings & Austin unpublished data). This suggests they are not pollen feeders. There are no published records of Pristaulacus visiting flowers, and no pollen has been found on the mouthparts of a range of museum specimens of Pristaulacus spp. from either North America or Australia (Jennings & Austin unpublished data). Until further field observations are made, the exact mode of feeding of aulacids remains unclear, although pollen analysis, together with observations on the structure of the mouthparts and recorded plant visitations, suggest that aulacids are nectar feeders.
Aulacus striatus appear to be univoltine (Skinner and Thompson 1960). Although there is no direct evidence to support this for other aulacids, data indicate that adults for most species fly during a relatively short period each year, suggesting they have an annual life-cycle. For example, A. pallipes Cresson has mostly been collected in the U.S.A. in July (Townes 1950), and the seasonal flight activity of A. burquei is in the period early May to late July and for A. lovei Ashmead late May to mid July (Smith 1996a). A few aulacids such as P. fasciatus, fly only in late summer in North America (Smith, pers.comm.)
Pristaulacus may be either univoltine or multivoltine depending on the species, although again there are very few studies other than dates of collection. Pristaulacus nigripes (Turner) apparently has an annual life cycle in northern India with emergence from May to September (Beeson 1941). Smith (1996) indicates that for P. flavicrurus (Bradley), flight activity takes place in June and July in North America, and for both P. strangaliae (Rohwer) and P. stigmaterus (Cresson) flights occur in May to the end of July. However, P. rufitarsis (Cresson) may have a life cycle that extends more than three years. Its cerambycid host, Saperda calcarata Say, has a life cycle from three to five years, depending on temperature (Anon. 1979). If P. rufitarsis emerges from the host larva only when it is fully grown and has chewed its way close to the surface of the wood, as is the case with A. striatus (Skinner and Thompson 1960; Askew 1971), then it too may take three to five years to complete its life cycle.
References
Anon. 1979. Poplars and Willows in Wood Production and Land Use. (FAO: Rome).
Askew, R. R. 1971. Parasitic Insects. (Heinemann: London).
Beeson, C. F. C. 1941. The Ecology and Control of the Forest Insects of India and Neighbouring Countries. (Government of India).
Benoit, P.L.G. 1984. Aulacidae, famille nouvelle pour la faune de l'Afrique tropicale (Hymenoptera). Revue de Zoologie Africaine 98, 799-803.
Benson, R. B. 1954. Classification of the Xiphydriidae (Hymenoptera). Transactions of the Royal Entomological Society of London 105, 151-162.
Bugnion, E. and Popoff, N. 1911. Recherches anatomiques sur Aulacus striatus Jur. (Hyménopt.); tube digestif, ovaires, oeufs pédiculés. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 12, 43-48.
Carlson, R. W. 1979. Superfamily Evanioidea. pp. 1109-1118. In Krombein, K. V., Hurd, P. D., Smith, D. R., and Burks, B. D. (Eds.), Catalog of Hymenoptera in America North of Mexico. Vol. 1. Symphyta and Apocrita (Parasitica). (Smithsonian Institution Press: Washington, DC).
Crosskey, R. W. 1951. The morphology, taxonomy, and biology of the British Evanioidea (Hymenoptera). Transactions of the Royal Entomological Society, London 102, 247-301.
Deyrup, M. A. 1984. A maple wood wasp, Xiphydria maculata, and its insect enemies (Hymenoptera: Xiphydriidae). The Great Lakes Entomologist 17, 17-28.
Dowton, M. and Austin, A. D. 1994. Molecular phylogeny of the insect order Hymenoptera: Apocritan relationships. Proceedings of the National Academy of Sciences 91, 9911-9915.
Dowton, M. and Austin, A. D. 2001. Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita - evolutionary transitions among parasitic wasps. Biological Journal of the Linnean Society 74, 87-111.
Evenhuis, H. H. and Vlug, H. J. 1975. Aulacus striatus, parasiet van Xiphydria camelus. Entomologische Berichten 35, 58.
Finlayson, T. and Hagen, K. S. 1977. Final-instar larvae of parasitic Hymenoptera. Simon Fraser University, Pest Management Papers 10, 1-111.
Gauld, I. and Bolton, B. (Eds.) 1996. The Hymenoptera (2nd. ed.) (British Museum (Natural History): London and Oxford University Press: Oxford).
Gaulle, J. de 1908. Catalogue systématique et biologique des Hyménoptères de France. Extr. de la Feuille des Jeunes Naturalistes) ( P. Klineksiek: Paris).
Giraud, J. 1877. Liste des éclosions d'insects observées par le Dr Joseph-Étienne Giraud ... recueillie et annotée par M. le Dr Alexandre Laboulbène. Annales de la Société Entomolgique de France (Ser. 5), 7, 397-436.
Györfi, J. 1964. The Hungarian species of the family Aulacidae. Annales Entomologica Fennici 30, 49-52.
Hedicke, H. 1939. Gasteruptiidae. Hymenopterorum Catalogus 11, 1-54.
Hedqvist, K. -J. 1973. Notes on the superfamily Evanioidea in Sweden with keys to families, genera and species (Hym., Apocrita). Entomologisk Tidskrift 94, 177-187.
Höppner, H. 1904. Zur biologie der Rubus-bewohner. Allegemeine Zeitschrift fur Entomologie 5/6, 97-103.
Jennings, J. T. and Austin, A. D. 2000. Higher-level phylogeny of the Aulacidae and Gasteruptiidae (Hymenoptera: Evanioidea). pp. 154-164. In Austin, A. D. & M. Dowton (Eds) The Hymenoptera: Evolution, Biodiversity and Biological Control. CSIRO Publishing, Melbourne.
Jennings, J. T. & Austin, A. D., 2004, Biology and host relationships of aulacid and gasteruptiid wasps (Hymenoptera: Evanioidea): a review. pp. 187-215. In Rajmohana, K., Sudheer, K., Girish Kumar, P., & Santhosh, S. (Eds.) Perspectives on Biosystematics and Biodiversity. University of Calicut, Kerala, India.
Jennings, J. T., Austin, A. D. & Stevens, N. B., 2004a, The aulacid wasp fauna of Western Australia with descriptions of six new species. Records of the Western Australian Museum 22, 115-128.
Jennings, J. T., Austin, A. D. & Stevens, N. B., 2004b, Species of the wasp genus Aulacus Jurine (Hymenoptera: Aulacidae) endemic to South Australia. Transactions of the Royal Society of South Australia 128, 13-21.
Jennings, J. T., Austin, A. D. & Stevens, N. B., 2004c, First record of Aulacidae (Hymenoptera: Evanioidea) from New Caledonia with descriptions of three new species of Aulacus Jurine. Journal of Australian Entomology 43, 346-352.
Jennings, J. T., Austin, A. D. & Stevens, N. B., 2004d, Hyptiogastrites electrinus Cockerell, 1917, from Burmese amber: redescription and its placement within the Evanioidea (Hymenoptera). Journal of Systematic Palaeontology 2, 127-132.
Jessop, J. P., and Toelken, H. R. (Eds.) 1986. Flora of South Australia, Part II. Leguminosae-Rubiaceae. (South Australian Government Printing Division: Adelaide).
Kieffer, J. J. 1912. Evaniidae. Das Tierreich 30, 1-431.
Lichtenstein, J. L. and Picard, F. 1918. Biologie des Pristaulacus Kieffer (Hym. Evan.) et leus répartition en France. Bulletin de la Société Entomologique France 1918, 109-110.
Madl, M. 1990. Eine neue Panaulix - Art Benoit aus Kenya (Hymenoptera, Evanioidea, Aulacidae). Mitteilungen der Munchner Entomologischen Gesellschaft 80, 85-88.
Mani, M. S., and Muzaffer, A. 1943. Studies on Indian parasitic Hymenoptera. III. - Descriptions of some new and records of some known Evaniidae. The Indian Journal of Entomology 5, 1-28.
Morley, C. 1916. Garden notes. Entomologist 49, 246-248
Muesebeck, C. F. W. 1958. Proctotrupoidea, pp. 88-93. In Krombein, K. V., ed. Hymenoptera of America North of Mexico, Synoptic Catalog, First Supplement. Agriculture Monograph No. 2, U. S. Department of Agriculture, Washington, DC.
Naumann, I. D. 1991. Hymenoptera. pp. 916-1000. In The Insects of Australia, Vol. II. (Melbourne University Press: Melbourne).
Oehlke, J. 1983. Revision der europäischen Aulacidae (Hymenoptera-Evanioidea. Beiträge zur Entomologie 33, 439-447.
Prinsloo, G. L. 1985. Order Hymenoptera (sawflies, wasps, bees, ants). Suborder Apocrita. Section Parasitica. pp. 404-406. In Scholtz, C. H., and Holm, E. (Eds.), Insects of Southern Africa (Butterworths: Durban).
Quicke, D. L. J., Fitton, M. G., Tunstead, J. R., Ingram, S. N., and Gaitens, P. V. 1994. Ovipositor structure and relationships within the Hymenoptera, with special reference to the Ichneumonoidea. Journal of Natural History 28, 635-682.
Ratzeburg, J. T. C. 1852. Ichneumon der Forstinsekten. 3, 21-22.
Riek, E. F. 1955. The Australian Xiphydriidae (Hyemnoptera Symphyta). Australian Journal of Zoology 3, 281-285.
Schmitschek, E. 1974. Beiträge zur ökologie von nadelbaum- und laubbaum-holzwespen (Hymenoptera, Siricidae). Zeitschrift für Angewandt Zoologie 75, 225-247.
Sedivy, J. and Capek, M. 1988. The species of Aulacidae in Czechoslovakia (Hymenoptera, Evanioidea). Acta Entomologica Bohemoslovaca 85, 231-233.
Short, J. R. T. 1952. The morphology of the head of larval Hymenoptera with special reference to the head of the Ichneumonoidea, including a classification of the final instar larvae of the Braconidae. The Transactions of the Royal Entomological Society of London 103, 27-84.
Short, J. R. T. 1959. The final instar larva of Aulacus striatus Jurine (Hym., Aulacidae)--a correction. Natural History Department, University of Aberdeen, pp. 217-219.
Skinner, E. R. and Thompson, G. H. 1960. Film: The Alder woodwasp and its Insect Enemies.
Smith, D.R. 1976. The xiphydriid woodwasps of North America (Hymenoptera : Xiphydriidae). Transactions of the American Entomological Society 102, 101-131.
Smith, D. R. 1979. Superfamily Siricoidea. pp. 125-137. In Krombein, K. V., Hurd, P. D. Smith, D. R., and Burks, B. D. (Eds.), Catalog of Hymenoptera in America North of Mexico. Vol. 1. Symphata and Apocrita (Parasitica) (Smithsonian Institution Press: Washington, DC).
Smith, D. R. 1996. Aulacidae (Hymenoptera) in the mid-Atlantic states, with a key to species of eastern North America. Proceedings of the Entomological Society of Washington 98, 274-291.
Smith, D. R. 2001. World catalog of the family Aulacidae (Hymenoptera). Contributions on Entomology, International 4, 263-319.
Townes, H. K. 1938. Pammegischia and Trichofoenus discarded (Aulacoid Hymenoptera). The Canadian Entomologist 70, 254-255.
Townes, H. K. 1950. The Nearctic species of Gasteruptiidae (Hymenoptera). Proceedings of the U. S. National Museum 100, 85-145.
Townes, H. K. 1951. Family Gasteruptiidae. pp. 655-661. In Muesebeck, C. F. W., Krombein, K. V., Townes, H. K., (Eds.) Hymenoptera of America North of Mexico, Synoptic Catalog, United States Department of Agriculture, Agriculture Monograph 2.
Walkley, L. M. 1952. An unusual aulacine from New Mexico (Hymenoptera - Gasteruptiidae). Proceedings of the Entomological Society of Washington 54, 185-186.
Title Illustrations

| Scientific Name | Aulacus sp. |
|---|---|
| Location | Australia |
| Specimen Condition | Dead Specimen |
| Identified By | John Jennings |
| Sex | Female |
| Life Cycle Stage | adult |
| View | lateral |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0.
|
| Copyright |
© John T. Jennings

|
About This Page
John T. Jennings

University of Adelaide, Glen Osmond, South Australia, Australia
Andrew R. Deans

Department of Entomology, NC State University
Correspondence regarding this page should be directed to John T. Jennings at and Andrew R. Deans at
Page copyright © 2005 John T. Jennings and Andrew R. Deans
 Page: Tree of Life
Aulacidae.
Authored by
John T. Jennings and Andrew R. Deans.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Aulacidae.
Authored by
John T. Jennings and Andrew R. Deans.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 06 October 2005
- Content changed 22 May 2006
Citing this page:
Jennings, John T. and Andrew R. Deans. 2006. Aulacidae. Version 22 May 2006. http://tolweb.org/Aulacidae/23534/2006.05.22 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site